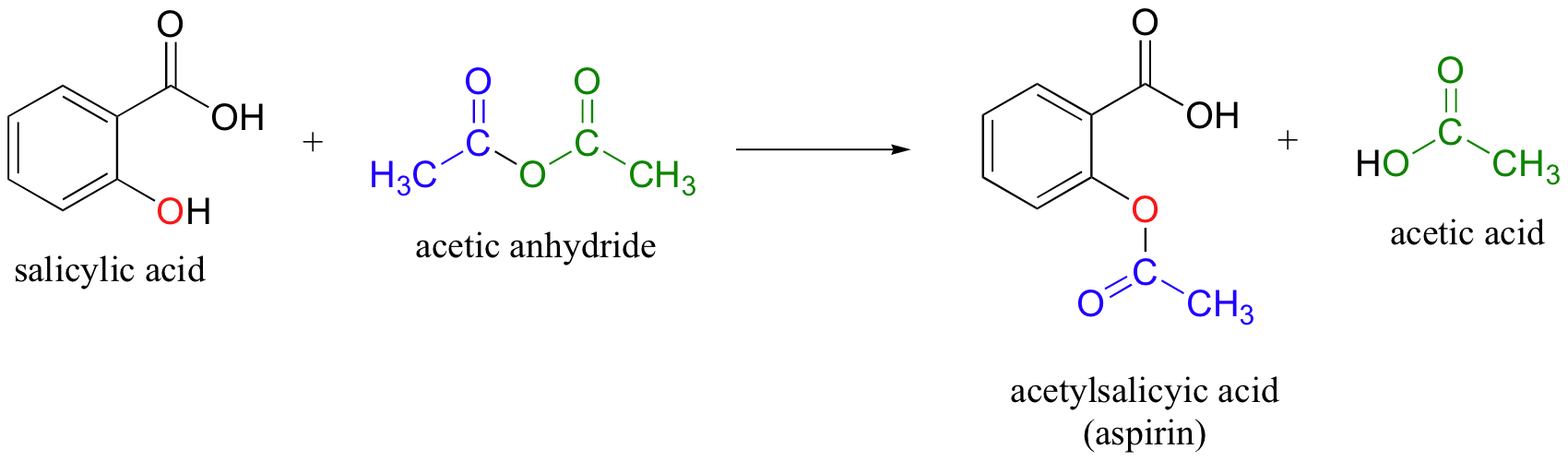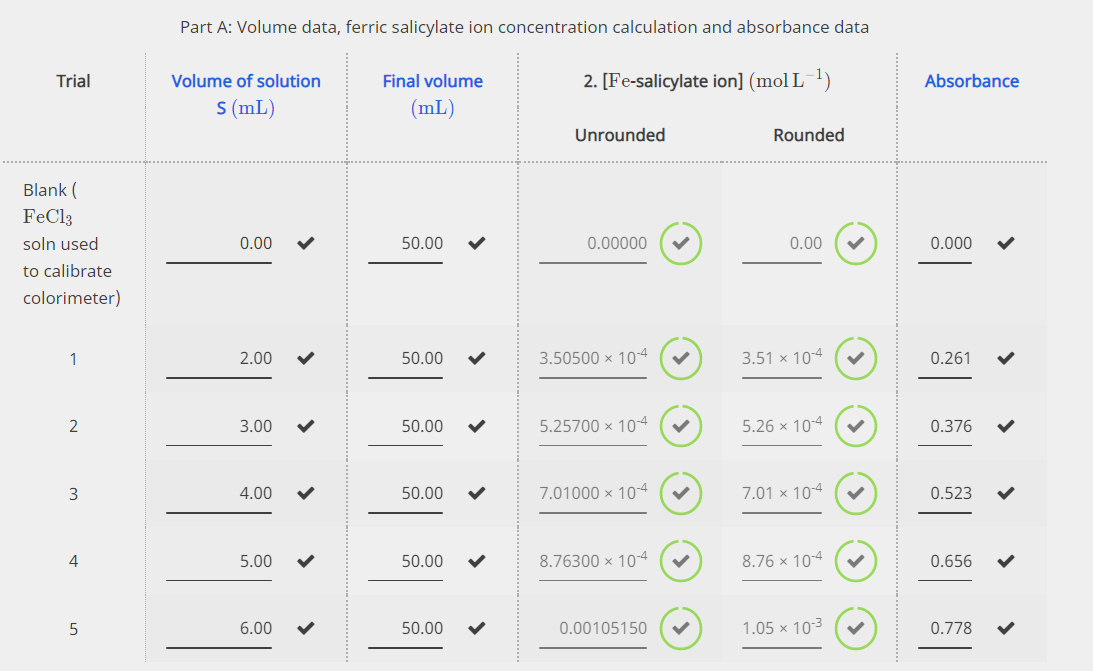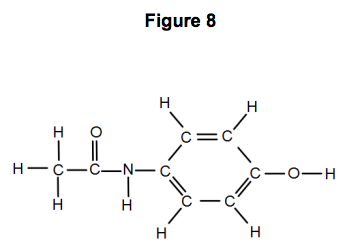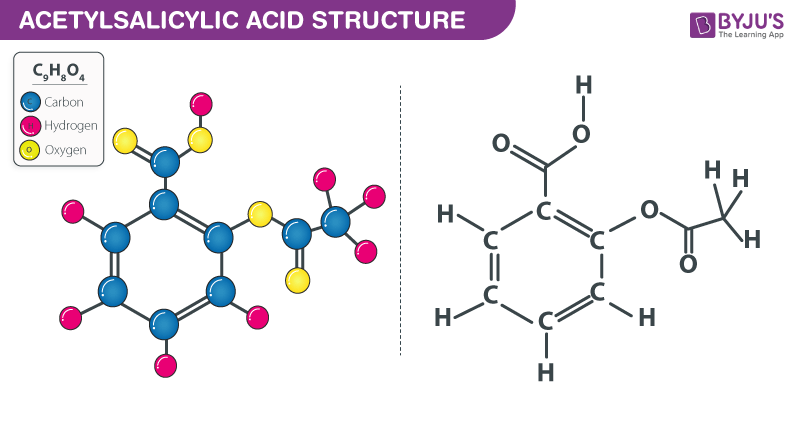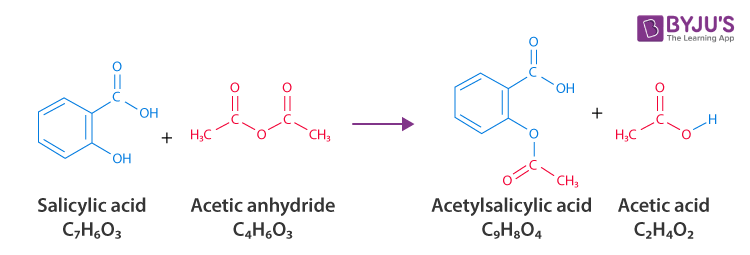
Acetylsalicylic acid (Aspirin ) - C9H8O4 - Formula, Structure, Properties, Preparation, Uses, Health risk and FAQs of Aspirin/ Acetylsalicylic ((C9H8O4)
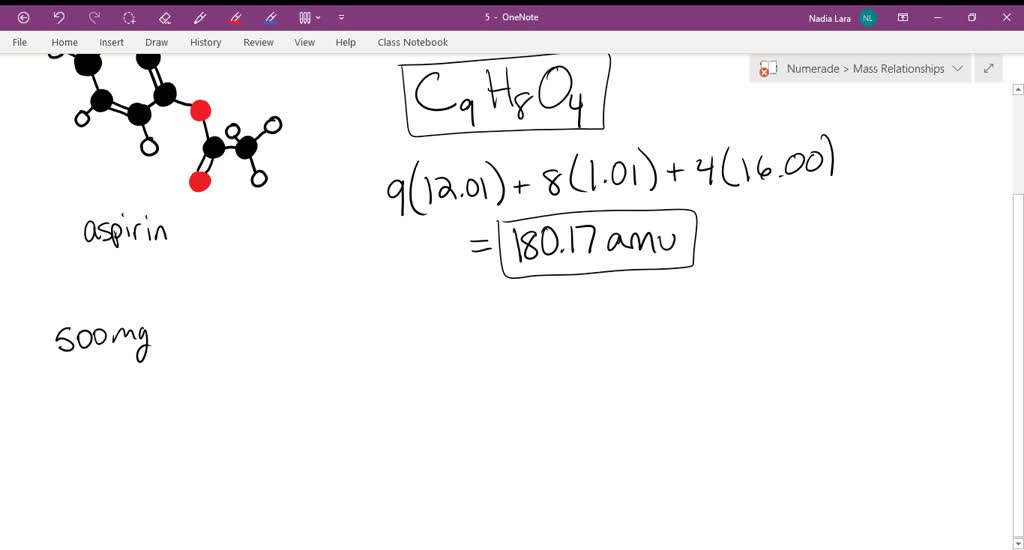
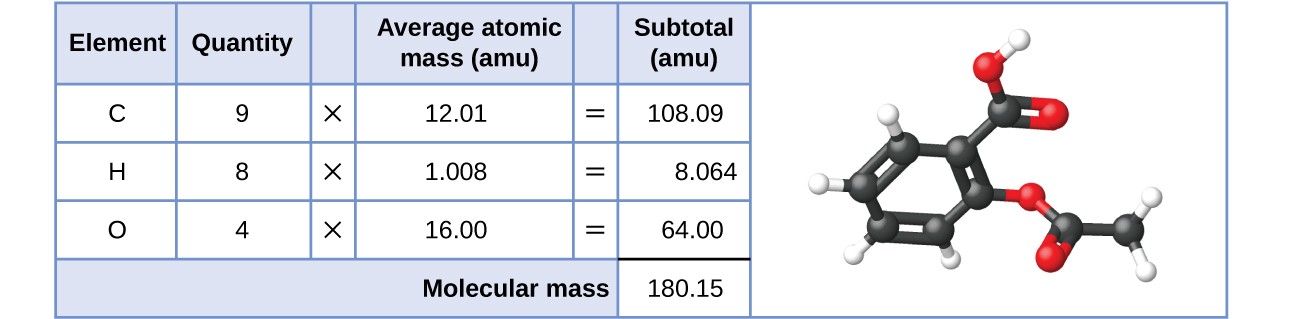
SOLVED:Aspirin can be represented by the adjacent ball-and-stick molecular model. Give the formula for aspirin, and calculate its molecular mass (red =O, gray =C, ivory =H ). How many moles of aspirin

SOLVED:The molecular formula of acetylsalicylic acid (aspirin), one of the most commonly used pain relievers, is C9 H8 O4 a. Calculate the molar mass of aspirin. b. A typical aspirin tablet contains

SOLVED: The molar mass of salicylic acid (CzH6O3) is 138 g/mole: The molar mass of [acetylsalicylic acid (CgH,O4) is 180 g/mole. Calculate the theoretical yield of acetylsalicylic acid (aspirin) based on thc
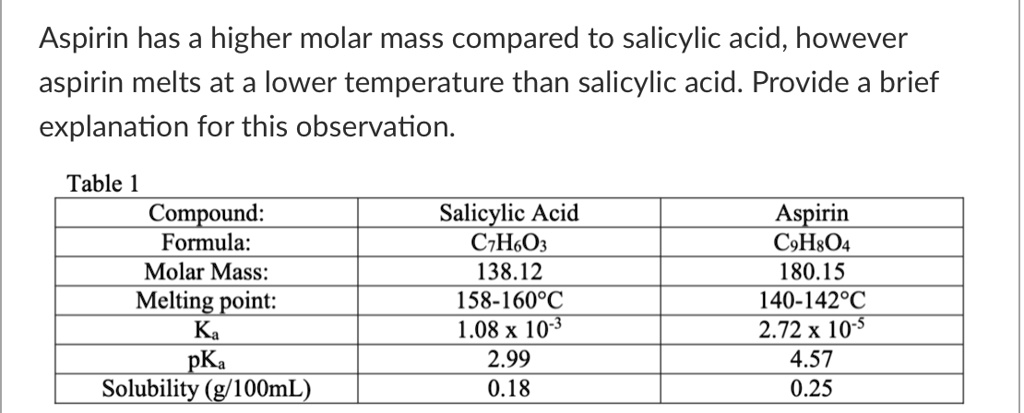
SOLVED: Aspirin has a higher molar mass compared to salicylic acid, however aspirin melts at a lower temperature than salicylic acid. Provide a brief explanation for this observation: Table 1 Compound: Formula: