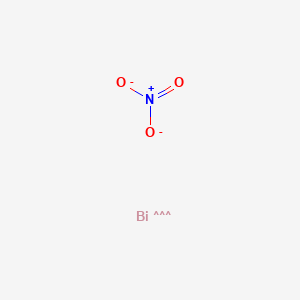
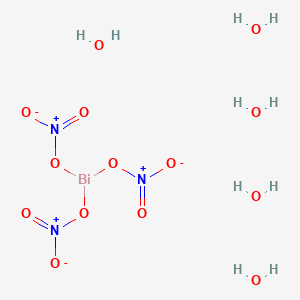
Plausible mechanism for Bi(NO3)2.5H2O catalyzed synthesis of 8-alkyl... | Download Scientific Diagram
![PDF] Highly Efficient Method for Synthesis of N-Amino-2-Pyridone Derivatives in the Presence of Catalysts such as Magnesium Oxide (MgO) and Bismuth(III) Nitrate Pentahydrate (Bi(NO3)3·5H2O) | Semantic Scholar PDF] Highly Efficient Method for Synthesis of N-Amino-2-Pyridone Derivatives in the Presence of Catalysts such as Magnesium Oxide (MgO) and Bismuth(III) Nitrate Pentahydrate (Bi(NO3)3·5H2O) | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/1425f000d5fbdea85cad84dd284dd3095f7c8f41/3-Table3-1.png)
PDF] Highly Efficient Method for Synthesis of N-Amino-2-Pyridone Derivatives in the Presence of Catalysts such as Magnesium Oxide (MgO) and Bismuth(III) Nitrate Pentahydrate (Bi(NO3)3·5H2O) | Semantic Scholar
The use of bismuth nitrate pentahydrate, Bi(NO3)3·5H2O, and bismuth subnitrate monohydrate, BiO(NO3)·H2O, for the preparation
Nitratopentakis(thiourea)bismuth(III) Nitrate M onohydrate, [Bi(NO3){ SC(NH2) 2 } 5](NO3)2.H 2 O, and Trinitratotris(thiourea)bi

Jual Bismuth ICP standard traceable to SRM from NIST Bi(NO3)3 in HNO3 2-3% 1000 mgl Bi CertiPUR® 100 mL Merck - PT. Mitra Tsalasa Jaya - Kota Tangerang Selatan , Banten | Indotrading

Gradual addition of KI solution to Bi(NO3)(3) solution initially produces a dark brown precipitate which dissolves in excess of KI to give a clear yellow solution. Write chemical equation for the above
2.H2O, and trinitratotris(thiourea)bismuth(III), [Bi(NO3)3{SC(NH2)2}3] - Jameson - 1984 - Acta Crystallographica Section C - Wiley Online Library Nitratopentakis(thiourea)bismuth(III) nitrate monohydrate, [Bi(NO3){SC(NH2)2 }5](NO3)2.H2O, and trinitratotris(thiourea)bismuth(III), [Bi(NO3)3{SC(NH2)2}3] - Jameson - 1984 - Acta Crystallographica Section C - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/5805be0b-9056-4de5-bd0b-85f7d4d9d668/s0108270184004108.fp.png)
Nitratopentakis(thiourea)bismuth(III) nitrate monohydrate, [Bi(NO3){SC(NH2)2 }5](NO3)2.H2O, and trinitratotris(thiourea)bismuth(III), [Bi(NO3)3{SC(NH2)2}3] - Jameson - 1984 - Acta Crystallographica Section C - Wiley Online Library

Hydrolysis Studies on Bismuth Nitrate: Synthesis and Crystallization of Four Novel Polynuclear Basic Bismuth Nitrates | Inorganic Chemistry

Gradual addition of potassium iodide solution to Bi(NO3)3 solution initially produces a dark brown precipitate which dissolves in excess of KI to give a clear yellow solution. Write the chemical equation for

Bi(NO3)3·5H2O: An efficient catalyst for one-pot synthesis of 3-((aryl)(diethylamino)methyl)-4-hydroxy-2H-chromen-2-ones and biscoumarin derivatives - ScienceDirect
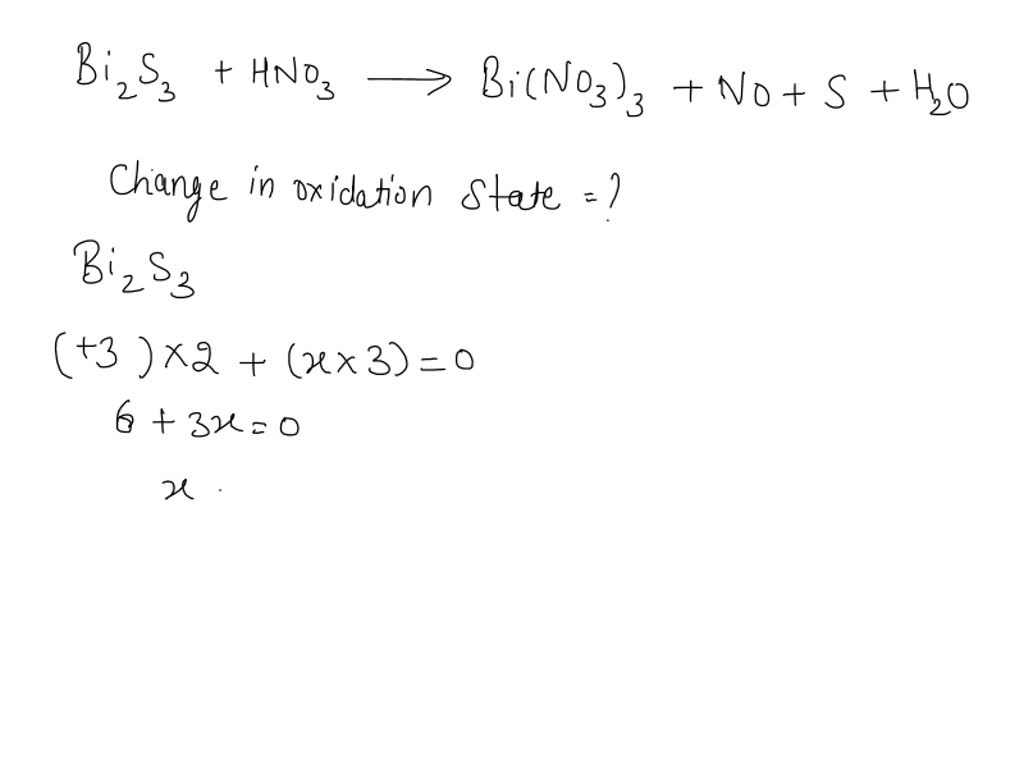
SOLVED: What is the change in the oxidation number of sulfur in the following reaction? Bi2S3(s) + HNO3(aq) → Bi(NO3)3(aq) + NO(g) +S(s) + H2O(l) A. –3 to +2, or +5 B. –

SOLVED: Write the net ionic equation for: 3 H2S(aq) Bi(NO3)3(aq) BizS3(s) HNO3(aq) Click here to enter oredit Your answer 3 Sz (a4)+2 Bi, (aq)-BizS , (s)






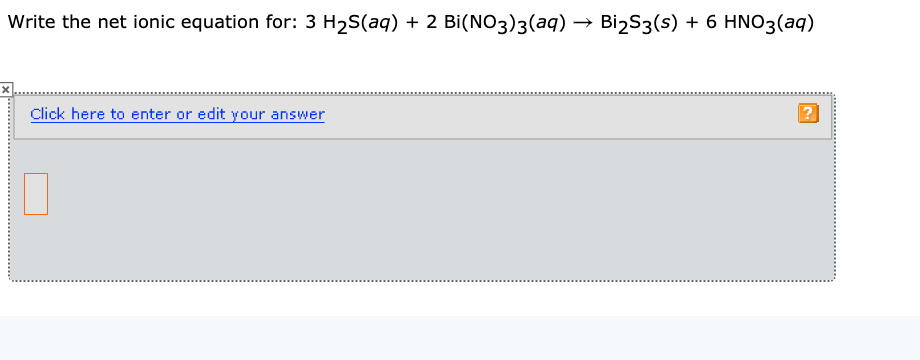

![Structure of [Bi(NO 3 ) 3 (H 2 O) 3 ]*18-crown-6. | Download Scientific Diagram Structure of [Bi(NO 3 ) 3 (H 2 O) 3 ]*18-crown-6. | Download Scientific Diagram](https://www.researchgate.net/publication/329597257/figure/fig2/AS:703233244790785@1544675279163/Structure-of-BiNO-3-3-H-2-O-3-18-crown-6.png)